Regenerative medicine refers to medical treatment that attempts to regenerate tissues and organs lost due to disease or injury and restore their functions. Until now, medical treatment has used artificial limbs, artificial joints, and transplants using other people’s organs, but these methods have many problems, such as rejection after organ transplant and ethical issues. On the other hand, researchers around the world are currently working hard on regenerative medicine, which overcomes the challenges of conventional medicine and opens up new treatment possibilities. In 2014, the world’s first transplant surgery of retinal pigment epithelial cells created from iPS cells was performed, a major step forward in opening the door to the next generation of regenerative medicine. Clinical studies are planned for Parkinson’s disease and heart failure patients, and there are high hopes that in 20 years it will be possible to create the heart, kidneys, and other organs themselves. One particularly promising area of recent regenerative medicine is the use of specialized cells called stem cells, with particular focus on somatic stem cells, which exist in the living body. Treatment with somatic stem cells has shown great promise because it is said to be highly safe and there are no ethical issues involved.
Our body is composed of 37 trillion cells, and stem cells are the source of producing those cells. They are present all over the body and produce each organ, blood, skin, etc. Stem cells have two unique abilities: the first is the ability to replicate cells exactly like themselves (self-renewal capacity), which allows stem cells to be maintained for long periods of time. Another is the ability to differentiate into various types of cells (pluripotency), which allows stem cells to generate new cells and regenerate tissues that have been damaged by disease or injury. At present, there are three main categories of stem cells that are being studied with an eye toward regenerative medicine. (1) Embryonic stem cells (ES cells), which are stem cells produced from fertilized eggs and have the ability to become all types of cells.
(2) “iPS cells,” which are established by inserting specific genes into cells extracted from body cells, such as skin, and can become any type of cell, just like ES cells. (3) Somatic stem cells, which are found in each tissue of the body and have the ability to differentiate into specific cells.
当クリニックでは、自由診療にて、高濃度ビタミンC療法を行っています。ぜひ一度お試しください。
高濃度ビタミンC療法は、次のような症状に効果が期待できます。症状、治療内容によって、ビタミンCの1回の投与量、回数は変わります。
当クリニックで使用している高濃度ビタミンCは、酸化防止剤を不使用です。米国メリット社製より、正規代理店を通して輸入しているため、品質に関してもご安心いただけます。
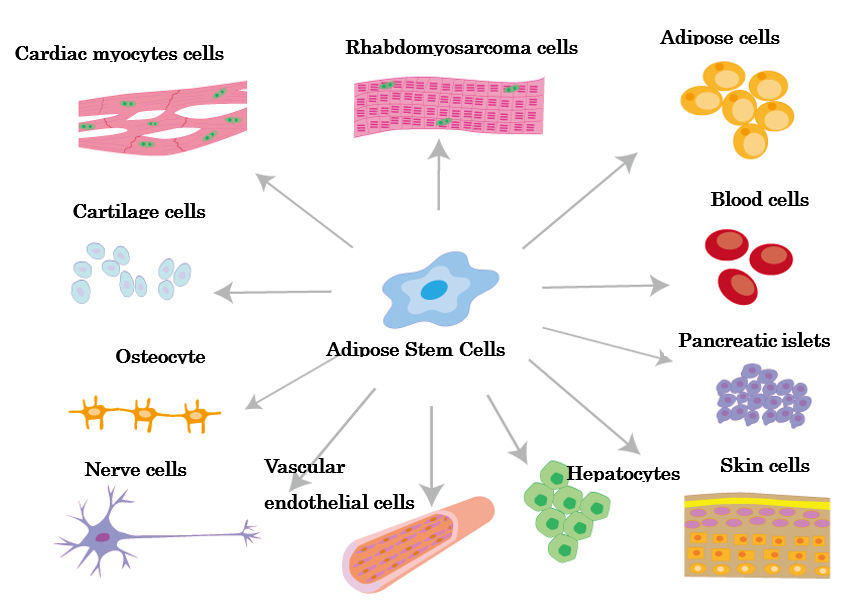
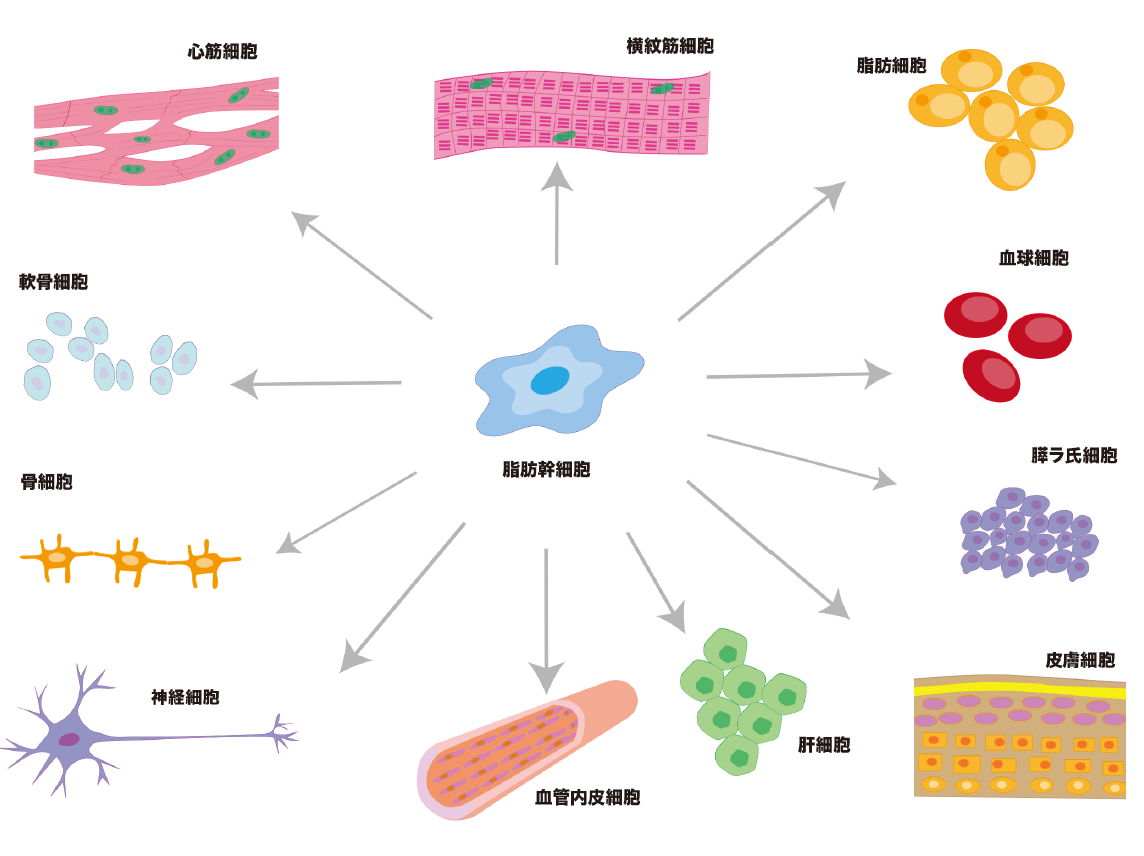
High potential Adipose stem cells
Stem cells have the ability to differentiate into various cell types
Of these stem cells, somatic stem cells are the most advanced in terms of practical application. Unlike artificially created ES and iPS cells, somatic stem cells actually work within our own bodies. For example, “hematopoietic stem cells”, which produce blood cells such as red blood cells, white blood cells, and platelets; “neural stem cells”, which produce nerve cells; and “mesenchymal stem cells”, which are believed to be capable of producing various tissues such as bone, cartilage, fat, and nerve.
Among mesenchymal stem cells, the discovery of adipose hepatocytes in adipose tissue in 2001 proved that adipose hepatocytes are 100-1000 times more easily obtained than bone marrow stem cells, and that they have the ability to differentiate into bone, cartilage, cardiac muscle cells, and blood vessel forming cells as well as adipose tissue. The main functions of adipose stem cells include injured wound healing, differentiation, immunomodulation, and neovascularization, and they have already been applied to the treatment of various diseases such as diabetes, myocardial infarction, cerebral infarction, liver dysfunction, allergic diseases, degenerative inflammatory diseases (knee osteoarthritis), autoimmune diseases (rheumatoid arthritis), and lung fibrosis, and their therapeutic effects have begun to be reported. Adipose stem cells are safer because they are the only autologous transplant, unlike umbilical cord, dental pulp, and amniotic stem cells, which are discussed below. Other mesenchymal stem cells would include umbilical cord stem cells, dental pulp stem cells, and amniotic stem cells. Umbilical cord stem cells are more difficult to obtain stably than adipose stem cells, but they contain more cytokines with strong cell proliferative and anti-inflammatory effects than adipose stem cell culture supernatant solution. We are planning to adopt these cells at our clinic, although they will be transplanted from another family. Dental pulp stem cells are taken from deciduous teeth of children and are said to have high regenerative proliferative ability because they produce a lot of cytokines related to cell proliferation. It is said to be particularly high in cytokines involved in nerve regeneration, and is particularly good for cerebro-periodontal infarction, vascular disorders, and periodontal disease. Of course, they are transplanted from another family. Amniotic stem cells are also transplanted from other families, but they are said to be highly proliferative and immunosuppressive among mesenchymal stem cells. Their effects on inflammatory diseases are particularly noted. Our clinic plans to adopt them in the future. Bone marrow stem cells have a strong property of differentiating into nerve cells and are said to have a high capacity for nerve regeneration. However, because of the skill and experience required to collect bone marrow, the low content of stem cells, and the difficulty of stem cell culture techniques, they are not widely used in general clinics. They are mainly used as hematopoietic stem cells for the treatment of blood disorders.
Thus, mesenchymal stem cells other than adipose stem cells are transplanted from other families, and the risk of rejection reactions such as anaphylaxis is assumed, so their practical use in Japan has not progressed very far. However, since fetal and infant tissues are used, they have low immunogenicity and are in a state of immunological tolerance, so they are relatively safe and are expected to be used in Japan in the future.
In November 2022, our clinic submitted to the Ministry of Health, Labor and Welfare a Type 2 Regenerative Medicine Provision Plan for the treatment of osteoarthritis of the knee using cultured mesenchymal stem cells derived from autologous adipose tissue, and obtained a plan number.(NA8150002)
In October 2024, our clinic submitted to the Ministry of Health, Labor and Welfare a Type 2 Regenerative Medicine Provision Plan for the treatment of chronic liver dysfunction using cultured mesenchymal stem cells derived from autologous adipose tissue, and obtained a plan number.(PB3240169)
The flow of this treatment is described below.
Flow from initial consultation to treatment
- Before this treatment, patients must first be tested for infectious diseases (hepatitis B virus (HBV), hepatitis C virus (HCV), human immunodeficiency virus (HIV), human T-cell leukemia virus type 1 (HTLV-1), parvovirus B19, treponema pneumoniae (TPHA, RPR), and if all tests are negative, this treatment is available. If the results of the blood test show that processing work cannot be performed, the patient will be charged only the initial consultation fee and the cost of the blood test. (See below for the fees).
- Patients with malignant tumors are not eligible for this service.
- In some rare cases, we may not be able to perform the procedure if the patient is not in good physical condition or if the blood is in poor condition. In such cases, you may be asked to have your blood drawn again.
- • Patients are required to examine X-ray and MRI images of the knee joint for the treatment of osteoarthritis or abdominal ultrasound sonography, fibroscan and special blood test for the treatment of injured liver.
1X-ray examination
- confirms that the patient has osteoarthritis of the knee, grade 2-4 of the Kellgren-Lawrence classification.
2MRI (nuclear magnetic resonance)
- evaluates the degree of cartilage and meniscus damage in the joint.
Patients who wish to receive regenerative treatment for liver disorders will be asked to undergo a Saisei-no-Mori liver checkup at our hospital during their first visit.
The Saisei no Mori Liver Checkup includes the following tests:
1Blood test
- A comprehensive test of liver function. We also check for viral hepatitis, liver tumor markers, and liver fibrosis markers.
2Abdominal ultrasound sonography
- estimates the grade of fatty liver and is confirmed the existence of liver cirrhosis and tumor in the liver.
3Fibroscan
- calculates the amount of fat tissue and liver fibrosis.
4Adipose tissue collection
- An incision is made under local anesthesia and 1 to 2 g of fat tissue from the lower abdomen is harvested. The wound is sutured with absorbable sutures (no need to remove sutures).
Fat stem cells are cultured at a cell culture facility approved by the Ministry of Health, Labor and Welfare (about 1 month).
5Administration
- It is administered slowly over approximately 1 hour via intravenous drip injection.
For treatment details, please refer to ``Recommendations for the Saisei no Mori liver checkup to avoid overlooking fatty liver disease, especially the progression from NAFLD to NASH - Proposals from prevention to treatment.''
Currently temporarily suspended. Scheduled to resume soon